New Money from Old Drugs. Are children with muscular dystrophy being served by the free market or taken advantage of?
I suspect that it is just because people are paying attention, but reports of unexplainably excessive pricing of both new and old drugs keep coming too fast to keep up with. I recently published a list of 447 drugs whose prices doubled or more between 2011 to 2015. Even that list was incomplete! This week’s prize winner is Emflaza, a drug that was recently approved by the Food and Drug Administration (FDA) to treat Duchenne Muscular Dystrophy (DMD).
The price proposed by Marathon Pharmaceutical, LLC is $89,000 per patient per year. We may be getting desensitized to such patient-bankrupting offerings, but what makes Emflaza stand out from the offending crowd is that in Canada, where some of the original research appears to have been done, the same drug for the same disorder costs a dollar a pill or less. As noted in the Wall Street Journal, the price set by Marathon is 50 to 70 times what most U.S. patients currently pay to buy the drug (illegally?) from on-line pharmacies in the United Kingdom. The more I learned about Emflaza, the more troubled I became. Allow me to share some of my discomfiture with you.
Emflaza is the new American brand name of deflazacort, an old generic standby marketed elsewhere in the world as a generic or under many different brand names. Before I started to look up the drug, I expected to find one of the “biologicals: man-made versions of complicated natural proteins found in our bodies that effect fundamental biochemical and hormonal processes. Think of man-made human insulin as an example. Biologicals as a group are the most expensive drugs on the market. Important and even critical for many rare and deadly diseases, they are increasingly being offered to treat more common disorders such as high cholesterol. To my surprise, and as the generic name suggests, Emflaza (deflazacort) is just another of many varieties of corticosteroids that have been on the market for decades. Deflazacort has the same clinical activity and side effects as prednisone or prednisolone from which it is chemically derived. The Physician’s Desk Reference (PDR) for deflazacort gives a long list of indications for its use which appears to me to be identical with the indications for prednisone. Such disorders include anaphylaxis, asthma, rheumatoid arthritis, systemic lupus erythematosis, and inflammatory bowel disease. It should not escape your notice that prednisone is one of the most widely used drugs in the USA, and is dirt cheap. Any puzzlement you may be experiencing as to why Emflaza should be so expensive is understandable.
Treating a bad disease.
Duchenne muscular dystrophy is a rare but fatal inherited disease that has its clinical onset in early childhood. It is reported to occur in 1 out of every 5000 male births and is very rare in females. It is one of the diseases for which the late Jerry Lewis raised money in his telethons. Its most serious and apparent symptom is muscular weakness progressing to a point where the individual can no longer eat or breath. The heart and brain are also involved. Untreated, most children die in childhood. A truly effective treatment is probably going to require advances in genetic or gene therapy– indeed, several such approaches are already under investigation. (They will likely be expensive too.)
I am still getting up to speed on Duchenne and do not yet know how it came to be that anti-inflammatory steroids became to be used to treat that genetic disorder. Presumably steroids decrease the damaging inflammatory effects of the body’s response to injured or dying muscle fibers. A striking study conducted in Canada compared treating boys with Duchenne with deflazacort or “usual” treatment without steroids. No one was cured, but loss of muscle strength was delayed meaningfully. Such clinical trials that compare one drug against no drug, or against a drug already proven to be effective but hoping for better, are critically important and need to be done in a dependable manner. Once a treatment is deemed effective, it may become the standard of care. Incorrect assumptions about effectiveness of any drug or treatment can and have led to exposing patients to the risk and cost of treatment without the possibility of benefit. Unnecessary medicine– like unnecessary surgery– can never be defended. To belabor the point, it is important to recognize that in clinical research a proposed treatment can always make the patient worse. Sometimes the placebo is safer. (That is why the FDA and clinical scientists push back against “freedom-to-take-a-chance” legislation that allows taking unproven remedies before FDA approval.)
In the case of corticosteroids, there are not many drugs for which the adverse effects are more inevitable or serious. There is in my opinion no “safe” dose of steroids. High doses of prednisone can kill people, and even moderate doses shorten lives. Corticosteroids cause metabolic abnormalities such as osteoporosis or bone necrosis, obesity, cataracts, diabetes, predisposition to infection, poor wound healing, growth retardation, thin skin, and more. The list of significant side effects is one of the longest of which I am aware. To give steroids to children (or anyone) is a big deal. I make these points because there have been some claims that at equivalent anti-inflammatory doses, that deflazacort has somewhat less severe, or at least different side effects than prednisone or other steroids. In my training and teaching in the U.S., it has been assumed that at equivalent anti-inflammatory doses, the side effects of available corticosteroids are essentially the same. I am told that for whatever historical or clinical reason, deflazacort is the most commonly used corticosteroid in Canada overall. In the U.S., Duchenne is often treated with prednisone. Some, if not many families obtain deflazacort from Canada. If in their wisdom our legislature continues to block importation of legal drugs from Canada, please keep these children in mind should they choose to take deflazacort instead of prednisone.
Is deflazacort really better than prednisone for Duchenne?
To address this issue, I went to Cochrane Library, my Bible for the careful structured analysis of the medical literature comparing medical treatments. In February, 2016, Matthews et. al. updated earlier reviews that had shown that steroids improved muscle strength and function in Duchenne for up to two years, but that available evidence for long-term outcomes and the cumulative effect of side effects was limited. It had been “unclear whether different corticosteroids differ greatly in side effects,” and that there had been “very low quality evidence” from two studies that deflazacort appeared to cause less weight gain at one year than prednisone. This latter conclusion about possible differences in weight gain remained unchanged in the 2016 update. In my opinion, very low quality evidence is insufficient to justify an $89,000 price tag no matter how many free coupons are handed out.
The most recent direct comparison of deflazacort to prednisone available to me is a pre-publication copy dated Aug. 2016 of a paper by Griggs et.al. and sponsored by Marathon of 196 boys who were treated daily for 52 weeks with two different doses of deflazacort, or prednisone. Presumably this study was presented to the FDA as part of Marathon’s application. The principal findings included that all three regimes were effective compared to placebo in the first 12 weeks in terms of strength and function. This improvement persisted for the duration of the study during which the placebo-treated patients received active drug too. In terms of adverse effects, there was more weight gain in the prednisone group, but in the deflazacort group there was a decrease in growth rate, more cataracts, psychotic disorder, and more frequent Cushingoid appearance (round face).
There may well be meaningful trade-offs in side effects, but it occurs to me that the differences in weight gain might be due to relative differences in the corticoid equivalencies of the two drugs. These boys were given hefty daily doses of prednisone– 0.75 mg/kg. At this level, the archetypical adult of 70 kg (154 lbs.) would be receiving 49 mg of prednisone per day. That is a lot– certainly enough for major and even life threatening side effects to take effect in short order! I would like to see research that compared lower doses of prednisone, or intermittent dosing such as only on weekends that has not yet to my knowledge been shown to be less effective than taking daily steroids. With its 196 subjects participating, this would have been one of the larger clinical studies. I hope the information was put to good use.
Old studies recycled.
With respect to the pricing of Emflaza and any application made to the FDA, the most stunning finding in the Griggs paper is that the study was completed 22 years ago in 1995! Much has changed since then including diagnostic criteria for Duschenne. The study was funded and conducted by another company– Nordic Merrell Dow Research, Inc.– which for reasons that are unstated in the paper discontinued development of deflazacort for Duchenne in the United States. It is important to know why. Is it possible that Marathon did not have to pay a dime for what must have been a very expensive clinical trial? The Griggs paper is presented as if it was an the original publication. Is it possible that 196 boys were exposed to the risks of a clinical study for which the results were not published? That would be hard to imagine and I hope to be reassured. [Non-reporting would make a case for mandatory registration and reporting of all clinical trials no matter what the result.]
Certainly, Marathon did undertake to finance the preparation of the report. The lead author is a consultant to Marathon, and at least two co-authors are employees of that company. It is not known to me if any of the original investigators of 22 years earlier participated in the preparation of the 2017 report. In my opinion, it is always preferable that clinical studies and their evaluation are done by completely independent entities. Such matters would be of concern to me if I were wearing my journal-reviewer’s hat.
Even assuming that deflazacort is the drug of chose.
If deflazacort really does have a better side effect profile than other steroids, why have not U.S. clinicians pushed for its use in Duchenne or other disorders? Given that deflazacort has been around a long time, is not particularly difficult or expensive to manufacture, is already available from several suppliers around the world, and that the study of its clinical effectiveness in Duchenne has already been paid for; how is it that that desperate families were threatened with this astonishingly high price? The yearly cost of $89,000 would pay for a lot of trips to Disney World or otherwise enhance the quality of life of the children and their families facing the unimaginably difficult challenge of a lethal disease?
Regulations are there to be gamed.
Business interests and most of the rest of us love to complain about the burdens of regulation but have no reluctance to game regulations to our advantage. Marathon used the option of placing deflazacort on the “fast track” for FDA approval of a treatment of an orphan disease. This FDA option program was set up to provide incentives to manufacturers to develop or promote treatments for rare diseases for which the market would be small. I do not claim to be familiar with the criteria by which the FDA judges whether a proposed drug represents a breakthrough, or is innovative or essential. Deflazacort in my opinion, when compared to the current American options, seems to be none of these.
Why are the costs of some “orphan drugs” so high?
The costs of discovery, testing, manufacture, and marketing must be paid for somehow. If there are only a relatively few potential patients, the price to the individual will necessarily be high unless there is some sort of subsidy, or unless the cost is subsumed widely among broad-based insurance premiums or tax-supported public programs. This is simple arithmetic. It is the responsibility of the FDA to assure us that new drugs are safe and effective– but obviously not necessarily accessible or affordable. How much profit is reasonable? Who should share in the cost? I do not know the answers to these important social policy questions, but Marathon is soon going to be queried by people with clout. Is the price outrageous or not? How much did it cost Marathon to put a new name on an old drug? How much of the cost will be for promotion or bonuses?
Everything in medicine can have adverse effects.
As I learn about the process of drug approval and pricing, I find a strategy in play that seems to explain at least some of the gigantic price increases in old standard drugs such as antibiotics. My reader may or not be surprised that a great deal of what passes for standard of care in today’s medicine is not supported by rigorous scientific evidence. Many drugs came into use for various diseases before today’s standards of FDA approval existed. In what seemed to be a good idea at the time, and to back-fill evidence that the old drugs actually do what we think they do; the FDA allows a pharmaceutical company to take an old drug and do a clinical study to show that it is effective for at least one indication. As an incentive, the company gets a protected period of exclusivity and the opportunity to set a new price. Because a clinician can legally use a drug approved for one diagnosis for any off-label use they think is appropriate, the market for Emflaza is likely to be much larger than for Duchenne alone! At $63,0000 per year, Emflaza could be a blockbuster for Marathon, especially if they claim a better side effect profile than prednisone and that claim is believed by some. It is fair to say that the rocketing prices of some old drugs may be an unintended consequence of a regulatory change that was supposed to be a good thing. For other drugs, simple greed may be the driving force.
Not the first generic steroid to get a price boost.
I understand that deflazacort is not available in the U.S. in the usual legal ways. I looked at the Medicare Part-D utilization database and confirmed that deflazacort was not prescribed to any beneficiaries in 2014. Prednisone however, is one of the most frequently prescribed drugs to Medicare patients. In 2014 there were 9.8 million prescriptions and refills for prednisone, for 4.6 million patients, at a cost of $78.5 million. I was surprised to see a single brand name for prednisone. It is Rayos, a delayed-release formulation of prednisone. It was “approved” in 2012 by the FDA for a broad range of corticosteroid indications. In 2014 there were 983 prescriptions for Rayos costing $1.6 million to Medicare patients! This is 200 times more expensive than prednisone. I am willing to be reeducated, but as a former rheumatologist, the need for an extended-release form of prednisone is in my opinion questionable, and certainly not worth the expenditure of so much money! This practice of making small changes in formulation is allowable by the FDA and has been used before to extend the period of patent exclusivity or to dramatically increase the price of the drug. Justifiable practice or not? You decide.
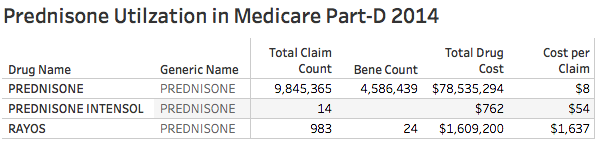 [The number of Rayos beneficiaries seems low but might be an artifact due to Medicare’s practice of suppressing beneficiary counts when the number of prescriptions is very low.]
[The number of Rayos beneficiaries seems low but might be an artifact due to Medicare’s practice of suppressing beneficiary counts when the number of prescriptions is very low.]
Who should the FDA work for?
Pessimist that I am, there is a potential darker side to all this. The FDA is a federal agency, and as such it is under the thrall of legislators and lobbyists who help finance reelections. The FDA gets leaned on all the time. I fear that the FDA has been the victim of regulatory capture– that is to say, overly influenced by the entities that it is supposed to regulate. It has been hard, for example, for the FDA to assemble a panel of experts to advise it who have not received money or its equivalent from the pharmaceutical industry. Concerns about regulatory capture was one of the concerns voiced when some years ago, the FDA began to receive much of its funding from industry fees instead of from the government. Does not the piper call the tune?
Story not yet over.
As of today, Marathon is holding back on its promotion of Emflaza because of resistance from concerned legislators and public interest groups. We will certainly learn more. The point I want to make here is that our system of development, approval, and promotion of drugs may not be working well to the benefit the people who need them. It doesn’t have to stay that way, but given the current political climate in Washington, I do not see things changing fast or at all.
If I have made an error in fact, help me fix it. I have never treated a person with Duchenne muscular dystrophy and my opinions come from the perspective of a generalist with a lot of experience with steroids!
Peter Hasselbacher, MD
Emeritus Professor of Medicine, UofL
Feb 17, 2017
Epilogue.
I have a personal as well as a policy reason for wanting more oversight and justice in the pharmaceutical marketplace. I have written before about my experiences with thyroxine to point out the craziness in the system. The $18 cash I had been paying for a three-month supply of my pills is much less than the copay I would have to pay for a single month if I processed the prescription through my own Part-D Medicare Drug plan. This time around, I noticed that the price was going up. I still got a discount, but I was told the full cash price would be around $120 for a month. I was told that the number of suppliers of thyroxine available to my pharmacy had just decreased from three to two. For the first time, my refill was dispensed in a bottle provided to my pharmacist by the manufacturer rather than in the usual undifferentiated orange plastic vial. Recall that thyroxine for thyroid replacement therapy is one of the very most frequently prescribed drugs for adults. Even modest price increases will earn some executive big bonuses! I hope that our representatives in Congress ask some hard questions of Marathon and any other drug company that raises prices in what a reasonable person might consider a high-handed manner.
[I still have a few links to insert.]

One thought on “Is Emflaza the Latest Drug Pricing Rip-off or Not?”
Comments are closed.